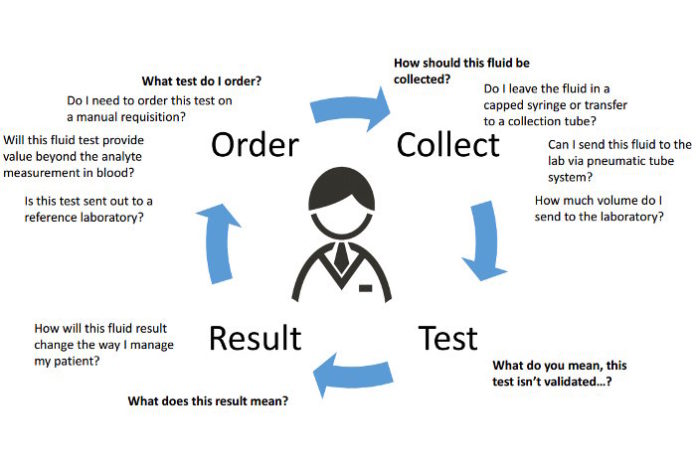
To identify the underlying cause of this accumulation, physicians commonly localize and remove fluid burden, send fluid samples to clinical laboratories for analysis, and offer insight at key points throughout the testing process as part of the diagnostic and prognostic evaluation (Figure 1). For their part, labs use several diagnostic tools to interrogate these samples, including measuring and identifying cells and biochemical analytes, and incubating cultures. Collecting a matched blood specimen and selecting tests appropriately also are key components of the work-up.
The Clinical and Laboratory Standards Institute (CLSI) is in the process of finalizing the second edition of the guideline document, Analysis of Body Fluids in Clinical Chemistry (1). This update covers topics across the total testing process, equipping laboratories with step-by-step instructions to assess the analytical performance of assays used to measure biologically important analytes in body fluid matrices. Additionally, this edition will provide laboratorians with an approach to identify and navigate past body fluid testing-related challenges and nuances.
The following case study and discussion provides an example not only of how physicians use body fluid results to diagnose, treat, and manage patients but also the role of clinical laboratories in supporting patient care.
Case Report
A 66-year-old female presented to the emergency department (ED) complaining that she was having difficulty breathing on exertion. This patient denied any symptoms of nausea, vomiting, hemoptysis, fever, chills, cough, or abdominal pain. Ten years previously she had had a renal transplant, but with immunosuppression her kidney function was stable. Within the past 2 weeks she had undergone cholecystectomy due to acute cholecystitis and cholelithiasis with calcification, and had experienced minor complications, including increased abdominal pain and minor fluid buildup in her right upper quadrant. However, these symptoms had resolved by post-operative day 6, and she was discharged home.
Presenting at the ED on post-operative day 13, her physical exam revealed bilateral pitting pedal edema, and her complete blood count results showed decreasing hemoglobin, indicating anemia of unknown etiology. Clinicians ruled out the possibility of deep vein thrombosis in both legs after performing bilateral venous ultrasounds. However, computed tomography (CT) without contrast of the patient’s chest revealed that she had bilateral pleural effusions and increased fluid accumulation localized around her liver. Consequently, she was readmitted and prepped for fluid drainage. Doctors removed 15 mL of blood-tinged perihepatic fluid with no visible indication of bile and left in place a gravity drain. Over the next 24 hours 700 mL of fluid continued to drain and her dyspnea improved dramatically. In addition, her stool, urine, blood, and drain fluid cultures displayed no growth. After 5 days (post-operative day 18), doctors removed the patient’s drain and discharged her home on oral antibiotics.
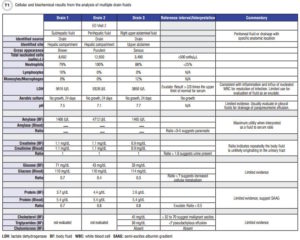
Nearly 3 weeks later, on post-operative day 36, the patient presented to the ED complaining of right upper quadrant abdominal pain and shortness of breath. CT examination revealed multiple fluid collections in her right abdomen and pelvis, as well as small bilateral pleural effusions unchanged from her prior CT exam. Doctors placed two drains in her hepatic compartment and removed 600–700 mL of cloudy bilious fluid from each. Endoscopic retrograde cholangiopancreatography (ERCP) revealed a bile leak and the patient was subsequently stented. Doctors placed a third drain the following day in the patient’s upper abdomen. Cellular and biochemical analysis revealed increased nucleated cells with neutrophil predominance, increased lactate dehydrogenase (LDH), and amylase enzymes in all three drain fluids (Table 1), suggestive of an inflammatory response likely due to a polymicrobial infection. Although the patient’s cultures were negative, her being discharged on oral antibiotics from her previous hospitalization was cited as the likely reason. The patient was discharged home still receiving IV antibiotics and with all three drains in place. Her fluid production continued to wane and eventually all three drains as well as her stent were removed. Ultimately, the patient’s immunosuppressed state secondary to renal transplant likely contributed to the continued recurrence of the abdominal infection.
Laboratories’ Role in Supporting Patient Management
As demonstrated by this case, clinical laboratorians are important partners in providing actionable results for patient management. However, laboratories generally are not responsible for collecting body fluids in the same way that phlebotomists collect blood. Moreover, body fluid testing is considered off-label use of assays according to most in vitro diagnostics manufacturers’ intended use claims. As cited by laboratory accrediting and regulatory agencies, laboratories should perform analytical validation for any test in which the specimen type being analyzed is outside the intended use statement. This places a significant burden on laboratories to know and control for pre-analytical conditions associated with fluid collection and to validate the performance of any assays for which they intend to offer testing. Until recently, the studies a laboratory might conduct to demonstrate adequate performance on alternate matrices have not been well described (1, 2).
Turning body fluid challenges into lessons learned
Challenge 1: The source and site of fluid collection as designated in the ordering system becomes the fluid type. Due to a variety of factors, laboratorians may not always have a clear understanding of the specimen source. For starters, the laboratory may have had limited, if any, input into the system used to order body fluid tests, consequently these systems may include anatomic locations and fluid descriptions to identify the origin of fluids that do not match the lab system-defined specimen sources, and interpretation or clarification is required. Often, laboratories receive body fluid specimens labeled as drain fluid with little more description than the type of drain, such as “wound” or “Jackson Pratt,” sometimes abbreviated as JP. The actual site of collection—sometimes a better clue to the fluid’s identity—may be missing.
In the case we present in this article, two specimens arrived at the laboratory labeled with the exact anatomical location the body fluid originated from, “subhepatic” and “perihepatic.” These particular fluid types were not validated body fluid types and as such, the technologist performed serial dilutions to verify that the chemistry analytes demonstrated a linear response as a means to rule out the presence of matrix interference prior to releasing the results. In contrast, the sample from drain 3 labeled “right upper abdominal fluid” was tested without question or further work-up, as “abdominal” is a recognized synonym for peritoneal fluid and therefore was considered a validated source by this laboratory according to its standard operating procedures. Although all the specimens were drain fluids originating from the patient’s peritoneal cavity, the laboratory performed serial dilutions on two of the three because of the descriptions provided. This delayed the lab’s result reporting.
Lesson 1: Labs may choose to refrain from testing body fluid types from non-validated sources and sites. However, to support appropriate patient management, they may need to contact clinical teams for clarification. In our case above, the lab determined after minimal investigation that the fluids in question originated in the patient’s abdominal cavity and likely were peritoneal fluid. Laboratories should decide whether they will perform additional accuracy studies on fluids labeled with anatomic descriptions, as in our case, in which the fluids ultimately are determined to have originated from a site that has been validated. The site and source are a part of the physician’s order and should not necessarily be changed without question.
We suggest that laboratories collect data on body fluids submitted for analysis. This will enable them to ensure not only that the fluid types and their source and site can be easily designated on the order, but also that they match the validated body fluid test menu offered by the laboratory. The advantage of doing this for the laboratory is it allows technologists to easily identify whether or not a fluid is a validated type. The number of fluids requiring investigation and potentially unnecessary work-up (dilution, recovery evaluation) depends on available selections from the ordering system.
Challenge 2: Body fluid matrix is a lot like serum or plasma … right? In principle, a body fluid matrix consists of molecules and substances that surround the analyte of interest. In many cases, the analyte of interest comes from blood filtered through circulation, which should reflect the composition of serum or plasma. The concentrations of proteins, electrolytes, and lipids present in a body fluid may alter recovery and interfere with accurate measurement of analytes within the body fluid matrix. Similar to blood, unless investigated, the extent to which these molecules may interfere in a fluid matrix is relatively unknown and nearly impossible to predict.
Lesson 2: Interference evaluations are key components of the analytical body fluid validation plan to ensure accuracy of reported body fluid results. Recovery experiments facilitate understanding of how interferences impact accuracy. Studies can be conducted by creating a series of body fluid aliquots with increasing concentrations of an interferent which are compared to the results from an unaffected sample (1, 2). Additionally, labs might consider using visual or spectrophotometric assessment of the body fluid by an automated instrument to estimate the concentration of interferent. In the case we present in this article, the lab noted that fluid from drain 1 was brown, possibly indicating aged blood accumulating in the hepatic fluid pocket or the potential presence of bile. Laboratories should have a policy for how to handle reporting in the event of hemolysis (hemoglobin) or icterus (bilirubin) thresholds being exceeded. Some options include canceling the test, reporting the result with comment, or attempting to dilute the interferent to report a result.
Challenge 3: Laboratories do not control collection of body fluids, and this has several consequences. The relatively wide variety of providers collecting body fluids and other specimens increases the possibility that the volume of fluid and container submitted to the laboratory will vary. Not to mention what it might be called (see Challenge and Lesson 1).
Lesson 3: Containers used to send specimens to the laboratory and body fluid volumes provided may be a direct reflection of minimal fluid burden, what the medical team is able to collect, or lack of communication from the laboratory on the necessary volume required for testing. Laboratorians’ discussion and active dialog with providers responsible for collecting non-blood specimens should include a list of appropriate collection containers as well as the laboratory’s volume and information requirements for body fluid specimens. Communicating this information is key to obtaining the volume necessary to perform body fluid testing. Additionally, receiving specimens in an appropriate container ensures the test will not be canceled because it cannot be completed as received.
Challenge 4: Which tests are actually useful and need to be validated? Some body fluid tests are considered routine in a diagnostic evaluation, so clinicians expect swift return of results for patient management. The fluid cell count is a great example, as it serves as a screening test for infectious or malignant causes of fluid accumulation. However, in the case presented in this article, despite concern for potential bile leakage, bilirubin was never measured.
Lesson 4: Certain biochemical analytes are helpful in some body fluids, while others have limited to no demonstrated utility. In reviewing our presented case, all fluids demonstrated increased total nucleated cells with a predominance of neutrophils suggestive of infection. Additional biochemical testing supportive of infectious etiology revealed decreased glucose concentrations and pH; however, the exact decision point for an abnormal fluid-to-plasma ratio of glucose is not well defined, and pH has demonstrated utility only when measured in pleural fluids for this purpose (1, 3). These biochemical assessments were likely of limited significance and in this case, the cell count was the most helpful for diagnosis.
In our case, there was an early suspicion for a potential bile leak. Interestingly, clinicians did not order bilirubin testing, possibly due to the fluid’s gross appearance and the patient’s past medical history. The ERCP procedure on her second ED visit did in fact confirm a bile leak. Lipid analysis in peritoneal fluids helps differentiate malignant from non-malignant causes; however, its evaluation on a serous looking fluid (drain 3) was likely unnecessary in this case (1).
Keep in mind that body fluid results are best interpreted along with concurrent measurement in serum. For example, in our presented case amylase was measured repeatedly with no matched blood sampling. When trying to differentiate fluid of pancreatic origin, amylase fluid-to-blood ratios provide better interpretive information. Commonly, total protein and LDH are also assayed in tandem with body fluids. This is done routinely for classifying fluid as an exudate; however, application of Light’s Criteria is best reserved for diagnostic evaluation of pleural fluids (3, 4). For the patient in our example, since this fluid originated from her peritoneal cavity, it may have been more appropriate to first measure the serum-ascites fluid albumin gradient (4, 5).
Conclusions
Developing an appropriate test menu and validation plan enables laboratories to confidently offer a body fluid test menu to support clinical needs. Clinical laboratories should be aware of the total testing process and consult resources that critically review the clinical application and utility of analyte measurement in body fluids. With the release of the second edition of CLSI’s guideline, Analysis of Body Fluids in Clinical Chemistry, laboratories will have guidance to embark on a body fluid validation project to verify an assay’s performance in body fluid matrices and clinical utility of the testing menu (1, 2, 6).
Deanna H. F. Franke, MT(ASCP), PhD, DABCC, is a PhD technical specialist at Carolinas HealthCare System in Charlotte, North Carolina. +Email: deanna.franke@carolinas-healthcare.org
Darci R. Block, PhD, DABCC, is director of laboratory services and co-director of the Central Clinical Laboratory at Mayo Clinic in Rochester, Minnesota. +Email: block.darci@mayo.edu
References
- Clinical and Laboratory Standards Institute (CLSI). Analysis of body fluids in clinical chemistry. 2nd Ed. C49. Wayne, Pennsylvania: CLSI 2016 (expected).
- Block DR, Franke DDH. Quick guide to body fluid testing. Washington, D.C.: AACC Press 2015.
- Light RW. The Light Criteria: The beginning and why they are useful 40 years later. Clin Chest Med 2013;34:21–6.
- Tarn AC, Lapworth R. Biochemical analysis of ascitic (peritoneal) fluid: What should we measure? Ann Clin Biochem 2010;47:397–407.
- Block DR, Algeciras-Schimnich A. Body fluid analysis: Clinical utility and applicability of published studies to guide interpretation of today’s laboratory testing in serous fluids. Crit Rev Clin Lab Sci 2013;50:107–24.
- Hussong JW, Kjeldsberg CR. Kjeldsberg’s body fluid analysis. Singapore: ASCP Press 2015.
Source: AACC